Mining the human immune response for innovative monoclonal antibodies to combat the growing global health threats such as antibiotic resistance and viral pandemics.

AR-501 phase 2a Top line data for Cystic Fibrosis treatment Learn More
AR-301 phase 3 data for treatment Pneumonia Learn More
About Us
Aridis Pharmaceuticals, Inc. is a late-stage clinical development company leading the creation of transformative, first-in-class anti-infectives for life-threatening viral and bacterial respiratory infections. The company’s pipeline of novel mechanism antibacterial and antivirals, sprung from its proprietary technology platforms, are designed to combat the growing public health threat of viral pandemics and antimicrobial resistant (AMR) bacteria.
Aridis is currently trading on the OTC Expert Market. Stock quotes and trading are limited to qualified institutional buyers. Please consult your stock broker for stock quotes and trading.

Aridis Pharmaceuticals is a biopharmaceutical company focused on the development of novel, differentiated therapies for infectious diseases. Complementing the product pipeline is a disruptive platform technology to discover rare, potent human monoclonal antibodies from patients. Our scientists and leadership team have a proven track record of innovation and successful drug development of therapeutic candidates from early discovery to commercial implementation.
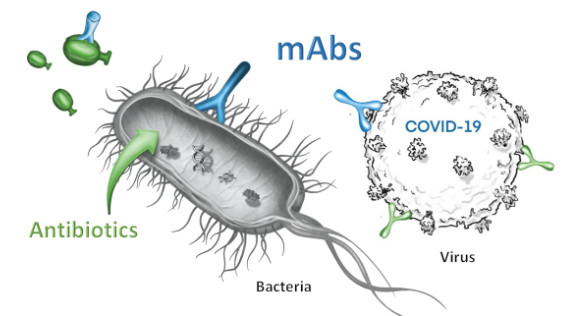
A core focus of our innovation is the discovery and development of monoclonal antibodies from convalescent infected patients. Monoclonal antibodies (mAbs) are proteins produced by our “humoral” immune system to neutralize the pathogens in a targeted fashion. Monoclonal antibodies exert their actions in multiple ways, all of which are largely distinct from antibiotics or antivirals, e.g. mAbs bind to the pathogen’s surface to block key functions, block entry into host cells, neutralize toxins, or to improve recognition by other immune cells.
Pipeline
Our products target infectious diseases that have a significant impact on life expectancy and address acute medical needs including:
- Hospital Acquired Infections (Ventilator Associated Pneumonia)
- Cystic Fibrosis
- Blood Stream Infections
- COVID-19 viral infection
AR-301 (SalvecinTM) is a fully human monoclonal IgG1 antibody that specifically targets S. aureus alpha-toxin, a toxic protein secreted by both methicillin-resistant S. aureus (MRSA) and methicillin-sensitive S. aureus (MSSA). AR-301 protects against alpha-toxin dependent destruction, preserving the human immune cells.
AR-501 is a novel small molecule anti-infective that diversifies our mAb immunotherapy-focused product portfolio. AR-501 is an inhalable form of gallium citrate, which acts as an iron analog to starve bacteria of iron. Gallium is believed to inhibit multiple iron-dependent synthetic and metabolic pathways required for pathogenicity. As with our monoclonal antibody programs, the mechanism of action of AR-501 is different from all antibiotics and is effective against antibiotic resistant bacteria.
AR-320 (suvratoxumab) is being evaluated as a pre-emptive treatment in S. aureus colonized, mechanically ventilated patients in the ICU. It is fully human monoclonal IgG1 antibody (mAb) that specifically targets S. aureus alpha-toxin, an important virulence factor that is secreted by both methicillin-resistant S. aureus (MRSA) and methicillin-sensitive S. aureus (MSSA). AR-320 is half-life extended, that is, able to sustain effective toxin neutralizing activities for approximately 3 months post-dose and above baseline level at one-year post-dose.
AR-701 is comprised of multiple fully human IgG1s monoclonal antibodies directed at conserved regions of the SARs-CoV-2 envelope proteins. AR-701 mAbs are designed to maintain broad coverage of SARs-CoV-2, including recently reported variants of SARS-CoV-2 such as the D614G variant, possible future variants of SARS-CoV-2.
Complementing our current clinical pipeline, we have two additional drug candidates in preclinical development. AR-401 is a mAb discovery program to treat infections caused by the Gram-negative bacterium Acinetobacter baumannii.
MabIgX® TECHNOLOGY
MINING THE HUMAN IMMUNE RESPONSE FOR NATURAL ANTI-INFECTIVES
In virtually all infectious diseases, there is a subset of disease-free individuals that may be generating rare, potent, protective immune responses to even the most lethal pathogens. Human monoclonal antibodies are often the key protective components. Our discovery technology platform aims to find and transform these components into innovative therapies to treat the masses.
News Releases
Welcome to the breaking news section for Aridis Pharmaceuticals. You can view ALL news by clicking button below.
Contact Us
Aridis Pharmaceuticals respects the privacy of our visitors to our website. By accessing or submitting information through our website, you agree to the terms of this Privacy Policy.


