
AR-101 (AerumabTM)
Fully Human mAb Against Pseudomonas aeruginosa LPS serotype O11
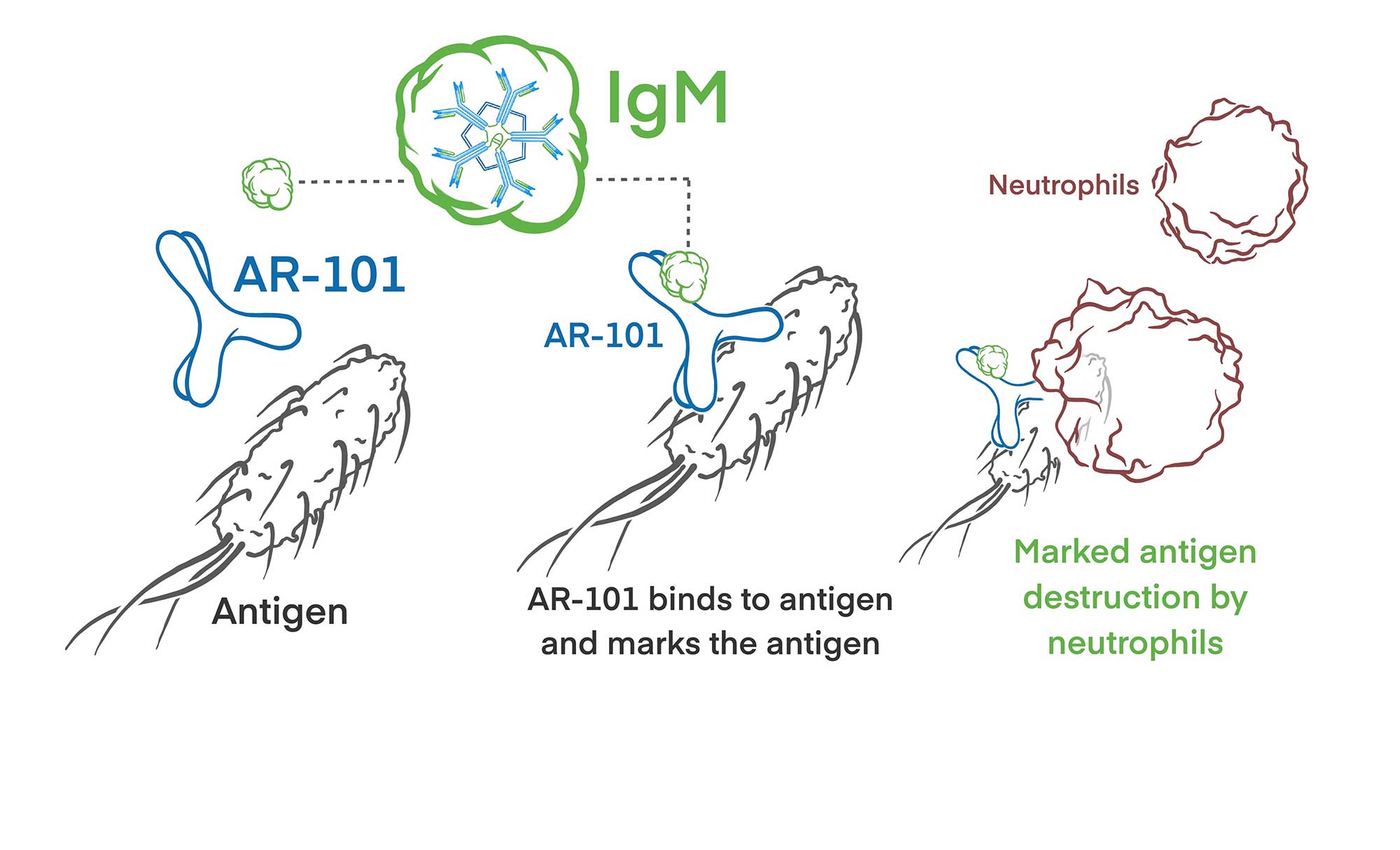
AR-101 (AerumabTM) is a highly specific monoclonal antibody targeted against P. aeruginosa lipopolysaccharide serotype O11, which accounts for ~22% of all P. aeruginosa hospital-acquired infections worldwide. Binding of AR-101 to P. aeruginosa bacteria facilitates human complement binding and improves immune recognition and destruction by circulating human phagocytes.
AR-101’s mechanism of action is distinct from mechanisms of antibiotic resistance, and is effective against multidrug resistant LPS serotype O11 P. aeruginosa clinical isolates. AR-101 is intended to be a first-line adjunctive therapy for patients with severe P. aeruginosa pneumonia being treated in intensive care units, and has orphan drug designation from the U.S. FDA and Europe’s EMA regulatory agencies.
AR-101 has successfully completed Phase 2a clinical testing in hospital-acquired pneumonia (HAP) and ventilator associated pneumonia (VAP) patients, demonstrating strong safety and efficacy trends. The Phase 2a clinical study evaluated AR-101 in 13 high risk HAP and VAP patients as an adjunctive therapy to standard of care antibiotics. AR-101 met the primary safety endpoints and showed a consistent trend toward improvement in mortality, shorter time to clinical cure of pneumonia, shorter time on mechanical ventilation, and fewer days in the ICU as compared to standard of care antibiotics-alone.
These top-line preliminary results underscore the strong potential of anti-infective monoclonal antibody therapies as an adjunctive drug to complement standard of care antibiotics.


