
AR-501 (Panaecin™)
Inhaled Gallium Citrate for Chronic Lung Infection in CF
AR-501 is a novel small molecule anti-infective that diversifies our mAb immunotherapy-focused product portfolio. AR-501 is an inhalable form of gallium citrate, which acts as an iron analog to starve bacteria of iron. Gallium is believed to inhibit multiple iron-dependent synthetic and metabolic pathways required for pathogenicity.
As with our monoclonal antibody programs, the mechanism of action of AR-501 is different from all antibiotics, and is effective against antibiotic resistant bacteria.
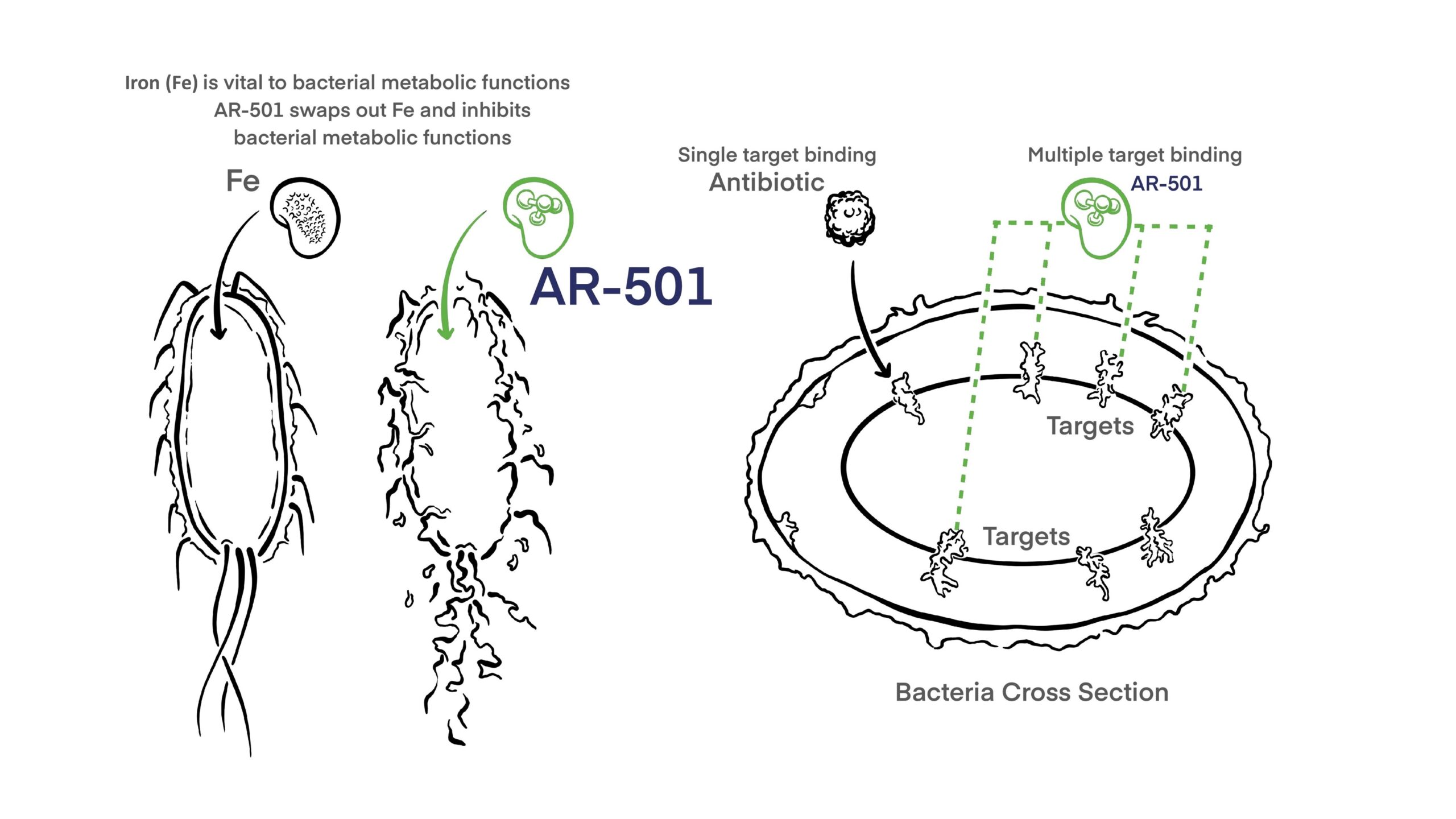
AR-501 has broad anti-bacterial activity against Gram-negative and Gram-positive bacteria in vitro, including antibiotic-resistant strains. AR-501’s inhibitory activity reaches bacteria growing in mature biofilms and prevents biofilm formation in viro. In mouse models of bacterial lung infections, a single inhalation exposure of aerosolized AR-501 protected the animal from morbidity and mortality. AR-501 was also protective when used in combination with antibiotics.
AR-501 is being developed as an inhaled treatment for the treatment of life-threatening bacterial lung infections in patients with cystic fibrosis (CF).
Intravenously administered gallium was found to be safe and efficacious in a recent Phase 2 clinical trial involving cystic fibrosis patients (the study was conducted by Univ. of Washington). However, intravenous formulation of gallium involves a 5-day continuous infusion, which is a demanding treatment regimen for the patients. AR-501’s inhaled dosage form can be self-administered within minutes, designed to be given once weekly, and allows for direct delivery of gallium to the lungs where the infections reside.
A Phase 1/2a clinical study in healthy adult volunteers and cystic fibrosis adult patients was recently completed. Inhaled AR-501 was well tolerated in health adult volunteers (n=48) through all repeated 5 weekly doses and at all dose levels tested (6.4mg, 20mg, and 40mg). No serious adverse events (SAE) were observed, and all adverse events (AE) were Grade 1 or Grade 2 in severity.
Top-line Phase 2a data in cystic fibrosis patients (n=41) showed that inhaled AR-501 was well tolerated through all repeated weekly doses and at all dose levels tested. No drug related serious adverse event (SAE) were observed and adverse events (AE) were mostly Grade 1 or Grade 2 in severity. Achieved high uptake of AR-501 in the respiratory tract, as measured by sputum drug concentrations, at levels that were more than 50-fold higher than that required for inhibition of the target bacteria P. aeruginosa. The level of AR-501 delivered to the lungs was also over 10-fold higher than achieved by intravenous (IV) administration in a Phase 2 study which showed lung function improvements in cystic fibrosis patients.


